Solutions-Flow Transmitter
Understanding the Basics of Groundwater Quality Monitoring Data
Understanding the Basics of Groundwater Quality Monitoring Data
Groundwater monitoring has always been a challenging and arduous task, yet it is essential for both human survival and natural development. In China, groundwater monitoring is still in its developmental stage. Today, I will introduce some fundamental knowledge related to current groundwater monitoring.
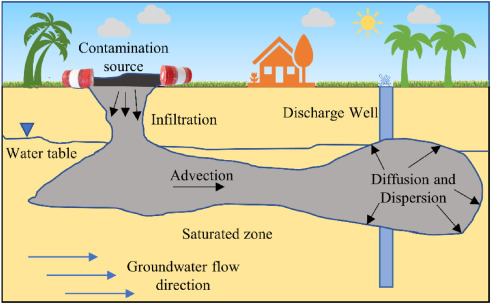
Indicator Classification
Groundwater monitoring involves hundreds of indicators, with different standards emphasizing different classifications of pollutants. By synthesizing authoritative documents such as the "Standards for Drinking Water Quality" and the "Standards for Groundwater Quality," monitoring indicators can be categorized into five major groups:
1. Sensory Indicators
Basic Assessment:
Color, turbidity, odor, etc., serve as the "physical examination" of water, providing a direct (intuitive, for clarity in English context, directly using the concept here) reflection of whether the water quality is abnormal.
Hidden Information:
Sudden changes in water turbidity or odor may indicate pollution events, such as industrial leaks.
2. Inorganic Pollutants
Common Members:
Heavy metals (arsenic, lead, mercury), nitrates, fluorides, etc.
Threats:
Long-term intake may lead to chronic poisoning, such as "dental fluorosis" caused by high-fluoride water.
3. Organic Pollutants
Stealthy Killers:
Pesticide residues (DDT, glyphosate), industrial solvents (benzene, vinyl chloride), etc.
Characteristics:
Difficult to degrade naturally, prone to accumulate in the human body, with a high risk of carcinogenicity.
4. Microorganisms and Radioactive Substances
Microorganisms:
Excessive E. coli may cause intestinal diseases.
Radioactive Substances:
Excessive uranium, radium, etc., can damage cell DNA and increase the risk of cancer.
5. Emerging Pollutants
Microplastics:
Derived from personal care products and plastic waste, potentially carrying toxins.
Drug Residues:
Antibiotics, hormones, etc., disrupting ecological balance.
Is the Data Reliable? Four Tips to "Debunk" False Reports
How can you verify the credibility of reports submitted by testing companies? The following methods can be used:
1. Cation-Anion Balance Method
Principle:
The total charge of cations (such as calcium, magnesium) and anions (such as sulfate, chloride) in water must be equal.
Error Threshold:
If the error exceeds ±5% (measured value) or ±10% (calculated value), the data may be falsified or key indicators may be missing.
2. Total Dissolved Solids (TDS) Verification
Calculated vs. Measured Values:
The error between the two must meet standards (e.g., when TDS > 1000 mg/L, the error should be < 5%).
Tips: There is a fixed ratio (0.55~0.75) between electrical conductivity and TDS. If the ratio is abnormal, the data may be problematic.
3. pH Value and Carbonates: A "Love-Hate" Relationship
Rule:
When pH < 8.34, carbonate (CO₃²⁻) should not be detected in water; conversely, if pH > 8.34, carbonic acid (H₂CO₃) should disappear.
Flaw:
If the report violates this rule, it indicates that the testing method or instrument may have errors.
4. The Secret of Sodium-Potassium Ratio
Truth in Natural Water:
In groundwater, sodium (Na⁺) content is usually much higher than potassium (K⁺).
Abnormal Signal:
If potassium suddenly "surpasses" sodium or the sum of sodium and potassium is zero, it is likely an analytical error!
RELATED NEWS
- Technical Introduction of Reverse Flushing Flow Meter 2025-06-12
- Liquid Level Switch, Level Meter And Liquid Level Transmitter 2025-05-22
- Troubleshooting Methods for Electromagnetic Flowmeters 2025-04-28
- Capacitive Level Gauges vs. Self-Powered Level Gauges 2025-03-19
- Customer Case Study:Chained Temperature Transmitters 2025-03-18
CATEGORIES
LATEST NEWS
CONTACT US
Contact:Roxy Deng
Phone:+8617794001501
Tel:+8617794001501
Email:roxy@besteetech.com
Add:Weibin District, Baoji, Shaanxi Province, China
 Roxy Deng
Roxy Deng Roxy Deng
Roxy Deng